Mimicking photosynthesis is not easy – but possible!
Making hydrogen fuel from water and visible light highly efficient
The bottleneck of artificial photosynthesis is visible light, because converting it into other forms of energy is not efficient. Researchers at Michigan Technological University have found a way to solve this issue, leading to an efficient technique to produce hydrogen fuel. Last week, the Journal of Physical Chemistry published their work.
The technique was developed by Yun Hang Hu, the Charles and Carroll McArthur professor of Materials Science and Engineer, and his PhD student, Bing Han, at Michigan Tech.
“Hydrogen is the future of cars,” says Hu. “And if you want to power hydrogen cars, you have to make hydrogen fuels.”
In this new hydrogen production process, the key is the interactions of a catalyst, light and a sacrificial molecule.
Playbook of Black Titanium Dioxide, Methanol and Light
As if in a complex sports game, the exchanges and counters between the materials used to split water look like a chemistry playbook. The players are black titanium dioxide (TiO2) and methanol (CH3OH) pitted against electron-hole recombination.
The goal of the game is to produce hydrogen molecules. Basically, that’s done by moving an electron from one place to another, like kicking a football to get a field goal. To make that score, a water molecule captures an electron excited within a material. When excited, electrons move up and down in different bands; the lower one here is the valence band and the higher one is the conduction band. The valence band and the conduction band are like goal posts, and between them is the band gap, which is like the playing field. The excited electron is the ball being passed around.
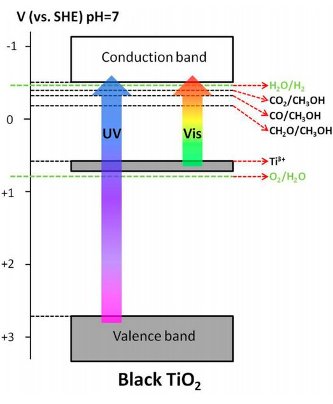
Source: Michigan Technological University
Solar energy, with both ultraviolet (UV) and visible light energy, is what gets this ball rolling. Light energy bounces off the first player, titanium dioxide, which is the material where the valence band and conduction band are in play. That excites an electron, making it a photo-excited charge that shoots up towards the conduction band. For UV light, the playing field is pretty big, and the band gap stretches 3.2 electron volts wide.
Here’s where the game gets more complicated, Harry Potter Quidditch-style, with balls that move quickly on their own. Electrons tend to zip like a golden snitch, and they sometimes misbehave, scurrying back to their starting place in the valence band, which negates the goal score and H2 production.
Enter player two: methanol. When the photo-excited electron shoots toward the conduction band, the methanol donates an electron via an oxidation reaction to swoop in and block the open spot left in the valence band. This sacrificial agent acts as a defense against the photo-excited electron snitching and scurrying its way back.
Playing in the Visible Light Spectrum
The play between titanium dioxide, methanol and UV light serves up hydrogen (H2) molecules. However, UV is only a very small part (4 percent) of solar energy. It is important to use visible light, since it constitutes about 45 percent of solar energy. Hu’s team has been able to increase the yield and energy efficiency up to two magnitudes greater than previously reported results using visible light instead of UV. But they had to change the playing field to do so.
“It’s a two-part process,” Hu says, explaining that the catalyst is black titanium dioxide (with 1 percent platinum), which attaches to a silicon dioxide substrate in their set-up. Hu’s team first generated a “light-diffuse-reflected surface” for the silicon dioxide, making it bumpy, which traps light waves and bounces them around. “We put the catalyst on the scattered surface, and it can increase the light absorption by one hundred times,” he explains.
Light absorption is an important step that generates photo-excited electrons, but it’s only part of what’s needed. Using visible light shortens the playing field, altering the band gap to only 1.3 electron volts in black titanium oxide. Specifically, that’s because an electron is excited to conduction band from Ti3+ energy level instead of the valence band, which is like starting at the 10-yard line instead of the 50-yard line.
The photo-excited electron has less distance to travel to the conduction band, but methanol doesn’t like to play along. The shortened distance from methanol to Ti3+ energy level greatly reduces methanol’s oxidation reaction, giving it less motivation to fill the leftover hole in the Ti3+ energy level. So, it needed a little heat in the second step of the process to get that reaction going again.
“We used temperature to increase the energy for methanol oxidation to donate electrons,” Hu says, adding that in the experiment they found 280 degrees Celsius to be the sweet spot.
With the modified silicon dioxide substrate and heat, Hu’s team also had to design a different set-up for the experiment. The water they used was steam, pushed into a chamber where it collided with a disk of roughened silicon dioxide studded with black titanium dioxide-platinum catalyst. Visible light then excited the electrons, and the hydrogen could then be syphoned off.
“The set-up is not complicated,” Hu says. “It’s actually convenient for scaling up commercially.”
Furthermore, this work created a hybrid process using both light and heat, which has opened a new door for visible light photosynthesis. The method is a big step closer to mimicking photosynthesis.
Source: Michigan Technological University, press release, 2015-08-19.
