Expanding the boundaries of CO2 fixation
Design and realization of synthetic enzymes open up an alternative to natural photorespiration
Photorespiration is a highly energy consuming process in plants that leads to the release of previously fixed CO2. Thus, engineering this metabolic process is a key approach for improvement of crop yield and for meeting the challenge of ever-rising CO2 levels in the atmosphere. Researchers led by Prof. Dr. Tobias Erb from the Max Planck Institute for terrestrial Microbiology have now succeeded in engineering the TaCo pathway, a synthetic photorespiratory bypass. This new-to-nature metabolic connection opens up newpossibilities of CO2 fixation and the production of value-added compounds.
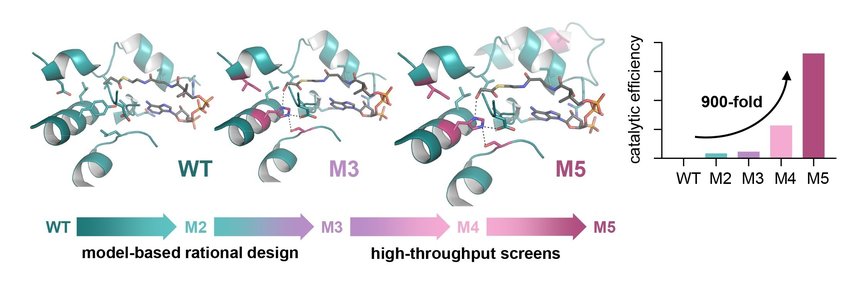
Engineering of glycolyl-CoA carboxylase (GCC), the key enzyme of the TaCo pathway. GCC was developed on the scaffold of a naturally occurring propionyl-CoA carboxylase (WT). Rational design led to the 50-times improved version M3, and additional high-throughput screening enabled the identification of a 900-fold improved version (M5). Max Planck Institute for terrestrial Microbiology/Scheffen
All life is dependent on the fixation of CO2 through plants. However, enzymatic efficiency of natural photosynthesis is limited, setting a boundary on agricultural productivity and CO2 fixation.Photorespiration is a detoxification process in plants that recycles a toxic by-product of photosynthesis, 2-phosphoglycolate. Photorespiration is highly energy consuming and leads to the release of previously fixed CO2, thus further curbing the photosynthetic balance.
Researchers led by Prof. Dr. Tobias Erb from the Max Planck Institute for terrestrial Microbiology have developed a synthetic photorespiratory bypass that represents an alternative to natural photorespiration. In collaboration with the group of Dr. Arren Bar-Even (MPI of Molecular Plant Physiology, Potsdam-Golm, and within the EU-funded project Future Agriculture, the team has designed the so-called tartronyl-CoA (TaCo) pathway that is much shorter than natural photorespiration and requires only 5 instead of 11 enzymes. The perhaps greatest benefit of the TaCo pathway is that it fixes CO2 instead of releasing it, as it happens in natural photorespiration. As a result, the TaCo pathway is more energy efficient than any other proposed photorespiratory bypass to date.
Building the TaCo pathway was a scientific journey that has led the researchers from computational model through enzymatic engineering, microfluidic high-throughut screening, cryo-EM-technology towards the successful in vitro implementation of a new-to-nature metabolic connection that opens up new possibilities for CO2 fixation and the production of value-added compounds. “The main challenge in realizing the TaCo pathway was to find all the required enzymes,” Marieke Scheffen, Postdoctoral researcher in Tobias Erb`s group and lead author of the study, recalls. “It meant that we had to look for enzymes that perform similar reactions and then “teach” them to perform the desired reaction.”
More efficient enzymes
For the TaCo pathway, initially a handful of enzymes was found that were able to catalyze the required reactions. However, they showed low catalytic efficiencies, meaning that they were quite slow compared to naturally occurring enzymes. The researchers aimed to boost especially the performance of the key enzyme of the TaCo pathway, glycolyl-CoA carboxylase (GCC), the catalyst that makes photorespiration carbon positive.
As a groundwork for creating a synthetic glycolyl-CoA carboxylase (GCC) the researchers developed a molecular model of the enzyme. Different variants of the enzyme were created based on a naturally occurring propionyl-CoA carboxylase, which is usually involved in fatty acid metabolism, as a scaffold by exchanging amino acid residues. This rational design strategy led to a 50-fold improvement of the enzyme’s catalytic efficiency with glycolyl-CoA.
In order to push the enzyme’s performance even further, the researchers teamed up with the group of Jean-Christophe Baret from the French National Centre for Scientific Research (CNRS, CRPP) Bordeaux, France, with whom they developed an ultrahigh-throughput microfluidic screen and screened thousands of synthetic variants. Within two rounds of subsequent microplate screenings, an enzyme variant was discovered that showed an even almost 900-fold increased catalytic efficiency with glycolyl-CoA. “With this catalytic efficiency, GCC is in the range of naturally occurring biotin-dependent carboxylases. This means we were able to engineer an enzyme from almost no activity towards glycolyl-CoA to very high activity, which is comparable to naturally evolved enzymes,” Marieke Scheffen explains.
High-resolution electron microscopy
Solving the molecular structure of this newly developed catalyst was achieved in yet another collaboration, with Jan and Sandra Schuller from the Max Planck Institute of Biochemistry, Martinsried (now SYNMIKRO in Marburg). The researchers applied cutting-edge cryogenic electron microscopy (cryo-EM) at an atomic resolution of 1.96 Å, thus pushing the limits of cryo-EM.
Finally, the synthetic GCC enzyme proved functional in in vitro experiments in combination with the two other enzymes of the TaCo pathway, thus forming an applicable carbon fixation pathway. “The TaCo pathway is not only a promising alternative for photorespiration”, says Group Leader Tobias Erb. “We could also show that it can be interfaced with other synthetic CO2 fixation cycles, like the CETCH cycle. Now we will be able to efficiently link synthetic CO2 fixation directly to central metabolism.”
This opens up a range of scientific possibilities, for example towards the recycling of polyethylene terephthalate (PET). The TaCo pathway could be used to convert ethylene glycol (a monomer of PET) directly into glycerate, making it usable for the production of biomass or value-added compounds. The next step will be to advance the in vivo implementation, in order to harness the full potential of the newly developed pathway.
Original publication
Scheffen, M.; Marchal, D.G.; Beneyton, T.; Schuller, S.K.; Klose, M.; Diehl, C.; Lehmann, J.; Pfister, P.; Carrillo, M.; He, H.; Aslan, S.; Cortina, N.S.; Claus, P.; Bollschweiler, D.; Baret, J.-C.; Schuller, J.M.; Zarzycki,J.; Bar-Even, A.; Erb, T.J.
A new-to-nature carboxylation module to improve natural and synthetic CO2 fixation
Nature Catalysis 1 (2021)
Source: Max Planck Institute for terrestrial Microbiology, press release, 2021-01-04.
