Berkeley Lab Scientists Teach Bacterium a New Trick for Artificial Photosynthesis
Functionality of biological systems with inorganic chemistry opens up for the next generation of advanced solar-to-chemical conversion technologies
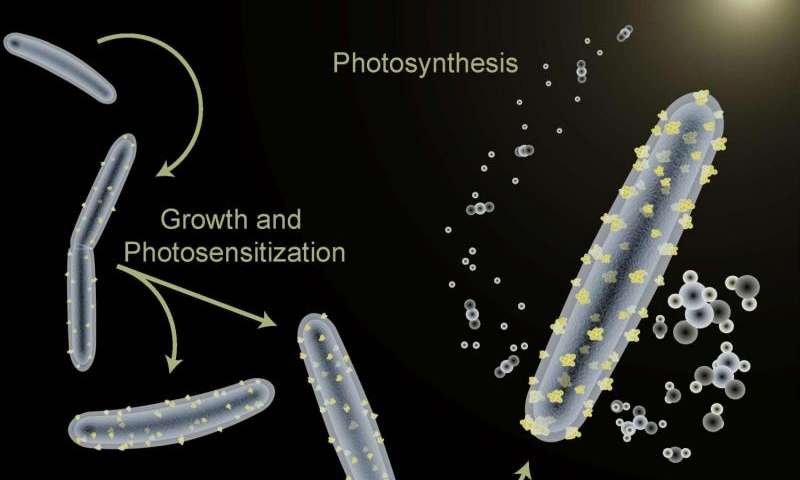
The bacterium Moorella thermoacetica is being used to perform photosynthesis in a hybrid artificial photosynthesis system for converting sunlight into valuable chemical products.
Trainers of dogs, horses, and other animal performers take note: a bacterium named Moorella thermoacetica has been induced to perform only a single trick, but it’s a doozy. Berkeley Lab researchers are using M. thermoacetica to perform photosynthesis – despite being non-photosynthetic – and also to synthesize semiconductor nanoparticles in a hybrid artificial photosynthesis system for converting sunlight into valuable chemical products.
“We’ve demonstrated the first self-photosensitization of a non-photosynthetic bacterium, M. thermoacetica, with cadmium sulfide nanoparticles to produce acetic acid from carbon dioxide at efficiencies and yield that are comparable to or may even exceed the capabilities of natural photosynthesis,” says Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division, who led this work.
“The bacteria/inorganic-semiconductor hybrid artificial photosynthesis system we’ve created is self-replicating through the bio-precipitation of cadmium sulfide nanoparticles, which serve as the light harvester to sustain cellular metabolism,” Yang says. “Demonstrating this cyborgian ability to self-augment the functionality of biological systems through inorganic chemistry opens up the integration of biotic and abiotic components for the next generation of advanced solar-to-chemical conversion technologies.”
Nanoscience expert Peidong Yang holds appointments with Berkeley Lab, UC Berkeley and the Kavli Energy NanoSciences Institute at Berkeley. (Photo by Roy Kaltschmidt)
Yang, who also holds appointments with UC Berkeley and the Kavli Energy NanoScience Institute (Kavli-ENSI) at Berkeley, is the corresponding author of a paper describing this research in Science. The paper is titled “Self-photosensitization of non-photosynthetic bacteria for solar-to-chemical production.” Co-authors are Kelsey Sakimoto and Andrew Barnabas Wong.
Photosynthesis is the process by which nature harvests sunlight and uses the solar energy to synthesize carbohydrates from carbon dioxide and water. Artificial versions of photosynthesis are being explored for the clean, green and sustainable production of chemical products now made from petroleum, primarily fuels and plastics. Yang and his research group have been at the forefront of developing artificial photosynthetic technologies that can realize the full potential of solar-to-chemical synthesis.
“In our latest study, we combined the highly efficient light harvesting of an inorganic semiconductor with the high specificity, low cost, and self-replication and self-repair of a biocatalyst,” Yang says. “By inducing the self-photosensitization of M. thermoacetica with cadmium sulfide nanoparticles, we enabled the photosynthesis of acetic acid from carbon dioxide over several days of light-dark cycles at relatively high quantum yields, demonstrating a self-replicating route toward solar-to-chemical carbon dioxide reduction.”
Cadmium sulfide is a well-studied semiconductor with a band structure and that is well-suited for photosynthesis. As both an “electrograph” (meaning it can undergo direct electron transfers from an electrode), and an “acetogen” (meaning it can direct nearly 90-percent of its photosynthetic products towards acetic acid), M. thermoacetica serves as the ideal model organism for demonstrating the capabilities of this hybrid artificial photosynthesis system.
“Our hybrid system combines the best of both worlds: the light-harvesting capabilities of semiconductors with the catalytic power of biology,” Yang says. “In this study, we’ve demonstrated not only that biomaterials can be of sufficient quality to carry out useful photochemistry, but that in some ways they may be even more advantageous in biological applications.”
This work was funded by the U.S. Department of Energy (DOE)’s Office of Science. The interface design part of the study was carried out the Molecular Foundry, a DOE Office Science User Facility hosted by Berkeley Lab.
Additional Information
For more about the research of Peidong Yang go here
About Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science.
About DOE’s Office of Science
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time.
Source: Lawrence Berkeley National Laboratory, press release, 2016-01-01.
