SwRI develops process to produce graphene from CO2
The internally funded project advances the lab-scale conversion of CO2 into graphene which is both valuable and useful for a variety of applications
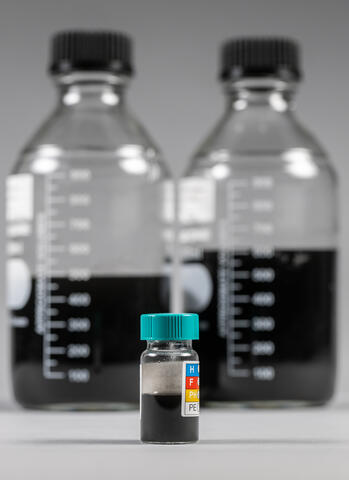
Chemical engineers at Southwest Research Institute produced gram quantities of graphene and other carbonaceous materials by bubbling carbon dioxide through a bed of liquefied alkali earth metals. Graphene, a carbon allotrope, is used for everything from biomedical devices to sensors and electronics. The internally funded project advances the lab-scale conversion of CO2 into graphene which, like diamonds, another form of carbon, is both valuable and useful for a variety of applications.
Many people are familiar with graphite, which is made of multiple layers of graphene, and often erroneously referred to as lead in pencils. Composed of carbon atoms arranged in a honeycomb-like lattice, graphene is stronger than diamonds, highly flexible, conductive and one of the lightest materials available. Its unique properties make it useful for coatings, lubricants and batteries, among other things.
“With almost unlimited potential uses for graphene, it’s understandable why the market continues to grow year over year,” said Miles Salas, the project lead. “We’re advancing this technology to support industrial clients looking for ways to create value-added products from their industrial CO2 waste.”
In the first phase of the project, SwRI scientists and engineers used a chemical reactor the size of a mini fridge to conduct a lab-scale experiment to gather data, test the graphene yield and identify the best reaction conditions.
“Redefining CO2 as a feedstock instead of a pollutant or sequestered product is key to increasing carbon capture projects around the globe,” said Michael Hartmann, manager of SwRI’s Carbon Capture and Utilization Process Development Section. “We are exploring graphene and other targeted CO2 utilization markets with a focus on scalability and commercialization pathways through lab-to-pilot demonstration projects.”
During phase two, engineers will create a small-scale pilot plant to further refine the process. For every 200 grams of alkali earth metal, which is inexpensive and abundant, the team can produce roughly 6 grams of graphene-containing material.
“Financial incentives for capturing carbon dioxide emissions are crucial. All solutions for reducing global CO2emissions must be economical or they are unlikely to be adopted. Providing a high-value product from CO2 will certainly drive support for future markets in carbon materials,” said Eloy Flores III, director of SwRI’s Chemical Engineering Department. “We continue to support efforts for industrial decarbonization in all areas to push the envelope in scaling new reactor designs for our government and industry clients.”
About Chemistry and Chemical Engineering at Southwest Research Institute
The staff develops technologies and performs services for the petroleum, chemical, power, and alternative fuels industries. With a “concept to commercialization” development process, we assist clients using process simulation, laboratory investigations, bench-scale development, and the design and operation of pilot and demonstration plants. Our laboratories are equipped for process, physical, chemical, and biological studies. We offer hydrocarbon characterization, physical property determination, and analytical testing. https://www.swri.org/what-we-do/technical-divisions/chemistry-chemical-engineering
Source: SwRI, press release, 2025-04-02.
