Scientists Identify Transition Metal for Highly-efficient CO2 Activation
Chinese study highlights the pivotal roles played by the M[η2-(O,O)C] species in CO2 activation
The atmospheric concentration of carbon dioxide (CO2) has been increasing over the past century, imposing severe consequences for global climate change and planetary temperature increase.
To reduce CO2 from the atmosphere and take it as the feedstock for sustainable energy sources, the capture and utilization of CO2 to create valuable chemicals is highly desired.
A research team led by Prof. JIANG Ling and Prof. FAN Hongjun from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. ZHAO Zhi from Hebei University of Engineering, characterized a transition metal M[η2-(O,O)C] species for highly-efficient CO2 activation.
The result was published in The Journal of Physical Chemistry Letters on Dec. 28.
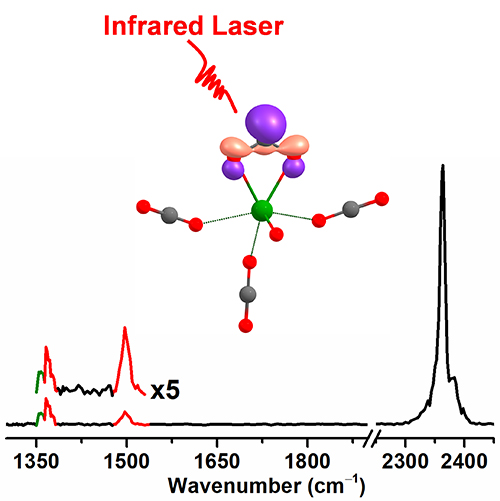
Spectroscopic Identification of Transition-Metal M[η2-(O,O)C] Species for Highly-Efficient CO2 Activation (Image by ZHENG Huijun)
The Zr[η2-(O,O)C] species yielded a CO2- radical ligand, showing high efficiency in CO2 activation. The CO2- radical and non-linear character of these series of M[η2-(O,O)C] complexes might enable high reactivity in many important reactions such as C-C coupling and C-H activation.
There were two important prerequisites for certain metals to form this intriguing M[η2-(O,O)C] species: the metal center had high reduction capability and the oxidation state of the metal center was lower than its highest one by one.
Systematic analyses for the effects of different transition and main-group metals on the formation of M[η2-(O,O)C] complexes provided comprehensive insights into the microscopic mechanism of CO2 activation by a single metal center, offering design criteria for single-atom catalyst with isolated transition metal atoms dispersed on supports. Such advances might be integrated into the CO2-activation and -utilization technology.
This study highlights the pivotal roles played by the M[η2-(O,O)C] species in CO2 activation and also opens new avenues towards the development of related single-atom catalysts with isolated transition metal atoms dispersed on supports.
Source: Dalian Institute of Chemical Physics, press release, 2021-01-05.
