Rice scientists, colleagues use doped graphene quantum dots to reduce carbon dioxide to fuel
Carbon dots dash toward ‘green’ recycling role
An illustration of a nitrogen-doped graphene quantum dot like those being tested at Rice University for use as catalysts to reduce carbon dioxide, a greenhouse gas, into valuable hydrocarbons. (Credit: Ajayan Group/Rice University)
Graphene quantum dots may offer a simple way to recycle waste carbon dioxide into valuable fuel rather than release it into the atmosphere or bury it underground, according to Rice University scientists.
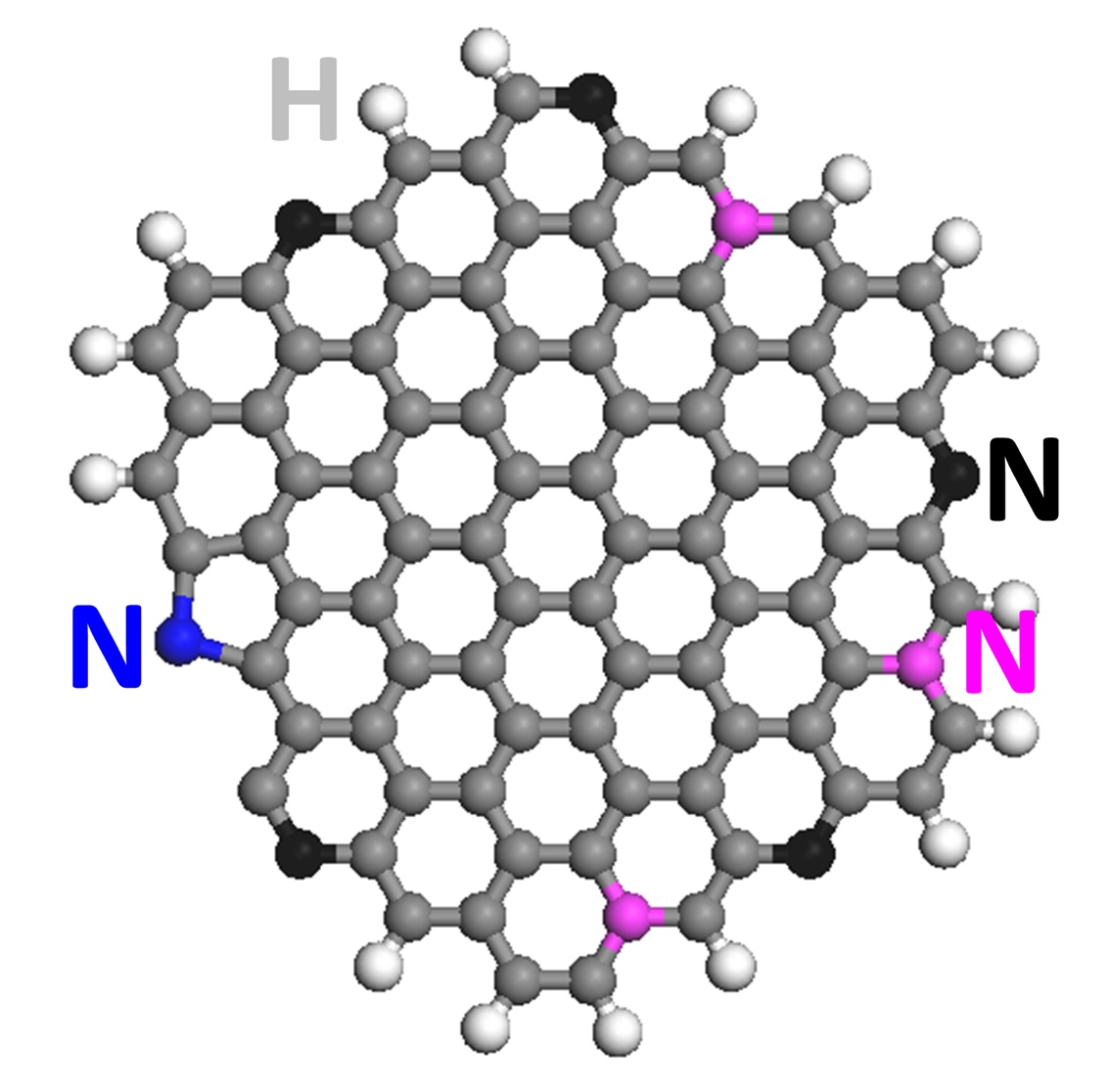
Nitrogen-doped graphene quantum dots (NGQDs) are an efficient electrocatalyst to make complex hydrocarbons from carbon dioxide, according to the research team led by Rice materials scientist Pulickel Ajayan. Using electrocatalysis, his lab has demonstrated the conversion of the greenhouse gas into small batches of ethylene and ethanol.
The research is detailed this week in Nature Communications.
Though they don’t entirely understand the mechanism, the researchers found NGQDs worked nearly as efficiently as copper, which is also being tested as a catalyst to reduce carbon dioxide into liquid fuels and chemicals. And NGQDs keep their catalytic activity for a long time, they reported.
“It is surprising because people have tried all different kinds of catalysts. And there are only a few real choices such as copper,” Ajayan said. “I think what we found is fundamentally interesting, because it provides an efficient pathway to screen new types of catalysts to convert carbon dioxide to higher-value products.”
Those problems are hardly a secret. Atmospheric carbon dioxide rose above 400 parts per million earlier this year, the highest it’s been in at least 800,000 years, as measured through ice-core analysis.
“If we can convert a sizable fraction of the carbon dioxide that is emitted, we could curb the rising levels of atmospheric carbon dioxide levels, which have been linked to climate change,” said co-author Paul Kenis of the University of Illinois.
In lab tests, NGQDs proved able to reduce carbon dioxide by up to 90 percent and convert 45 percent into either ethylene or alcohol, comparable to copper electrocatalysts.

A single nitrogen-doped graphene quantum dot with zig-zag edges. (Credit: Ajayan Group/Rice University) – Zoom -
Graphene quantum dots are atom-thick sheets of carbon atoms that have been split into particles about a nanometer thick and just a few nanometers wide. The addition of nitrogen atoms to the dots enables varying chemical reactions when an electric current is applied and a feedstock like carbon dioxide is introduced.
“Carbon is typically not a catalyst,” Ajayan said. “One of our questions is why this doping is so effective. When nitrogen is inserted into the hexagonal graphitic lattice, there are multiple positions it can take. Each of these positions, depending on where nitrogen sits, should have different catalytic activity. So it’s been a puzzle, and though people have written a lot of papers in the last five to 10 years on doped and defective carbon being catalytic, the puzzle is not really solved.”
“Our findings suggest that the pyridinic nitrogen (a basic organic compound) sitting at the edge of graphene quantum dots leads the catalytic conversion of carbon dioxide to hydrocarbons,” said Rice postdoctoral researcher Jingjie Wu, co-lead author of the paper. “The next task is further increasing nitrogen concentration to help increase the yield of hydrocarbons.”
Ajayan noted that while electrocatalysis is effective at lab scales for now, industry relies on scalable thermal catalysis to produce fuels and chemicals. “For that reason, companies probably won’t use it any time soon for large-scale production. But electrocatalysis can be easily done in the lab, and we showed it will be useful in the development of new catalysts.”
Co-lead authors of the paper are Sichao Ma of the University of Illinois at Urbana-Champaign and Kyushu University, Fukuoka, Japan, and Jing Sun of the Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences.
Co-authors are Jake Gold, Lingyang Zhu, Aaron Yu and Raymond Luo of the University of Illinois at Urbana-Champaign; Chandra Sekhar Tiwary of Rice; Byoungsu Kim of the University of Illinois at Urbana-Champaign and Kyushu University; Nitin Chopra and Ihab Odeh of SABIC Americas, Inc., Sugar Land, Texas; Robert Vajtai, a senior faculty fellow in materials science and nanoengineering at Rice; Jun Lou, a professor of materials science and nanoengineering at Rice; and Guqiao Ding of the Chinese Academy of Sciences. Kenis is the William H. and Janet G. Lycan Professor and head of chemical and biomolecular engineering at the University of Illinois at Urbana-Champaign with an appointment at Kyushu University.
Ajayan is chair of Rice’s Department of Materials Science and NanoEngineering, the Benjamin M. and Mary Greenwood Anderson Professor in Engineering and a professor of chemistry.
SABIC Global Technologies, B.V. supported the research.
About Rice University
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,910 undergraduates and 2,809 graduate students, Rice’s undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for happiest students and for lots of race/class interaction by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go to http://tinyurl.com/RiceUniversityoverview.
Source: Rice University, press release, 2016-12-16.
