Is CO₂ the Key to a More Sustainable Chemical and Fuel Industry?
Innovative Carbon Capture and Utilisation (CCU) technologies can convert captured CO₂ from industrial point sources and direct air capture into chemical base materials such as methanol, ethanol, ethylene or even polyols and other polymer building blocks
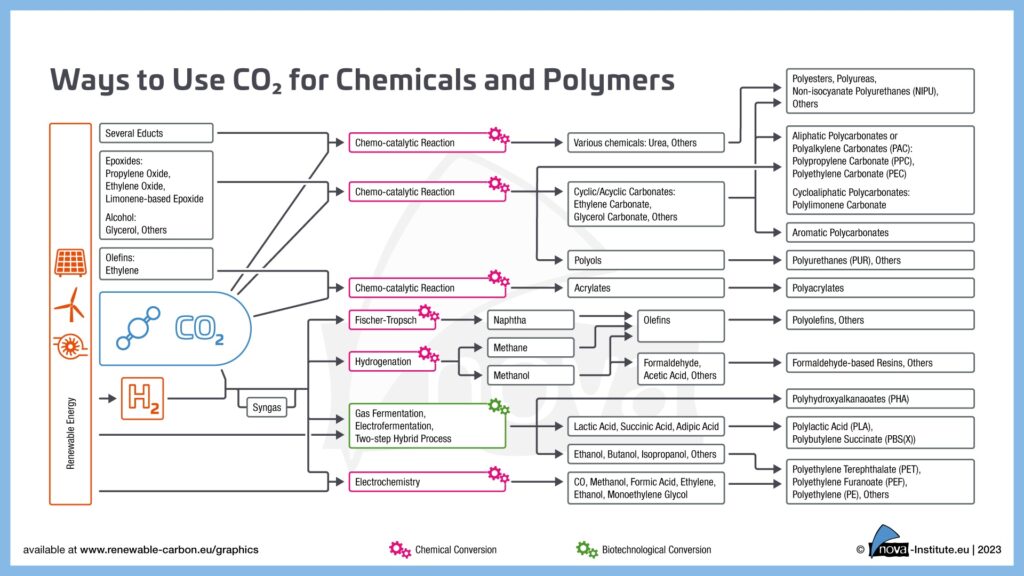
Carbon dioxide (CO₂) is often viewed solely as a major contributor to climate change and global warming, but it also holds significant potential as a valuable resource. Utilising CO₂ as a feedstock in chemical and fuel production could play a key role in transitioning to a more sustainable chemical sector and global economy. Innovative approaches focus on converting captured CO₂ into value-added products, such as fuels, chemicals, and materials, through various technical methods.
The chemical industry is a significant consumer of carbon, which to date has traditionally been generated from fossil sources such as oil, coal and natural gas. Alternative sources of carbon include biomass and CO₂ in particular, which offer more sustainable solutions. Innovative Carbon Capture and Utilisation (CCU) technologies can convert captured CO₂ from industrial point sources and direct air capture into chemical base materials such as methanol, ethanol, ethylene or even polyols and other polymer building blocks. These substances serve as the foundation for a wide range of products, including solvents, plastics, adhesives, paints and textiles.
Reduction of CO2 to syngas and other products
The production of base chemicals and fuels through CO₂-reduction involves converting carbon dioxide using external energy sources, primarily green hydrogen or direct electricity. When hydrogen is used, it is generated through water electrolysis powered by renewable energy (e.g., wind, solar), splitting water into hydrogen (H₂) and oxygen (O₂). Modern electrolysers achieve approximately 70% efficiency in converting renewable electricity into hydrogen, which can then be utilized to reduce CO₂ into a reactive mixture of CO and hydrogen, known as syngas. Syngas can undergo further processing through thermochemicalconversion technologies that use heat, pressure, and catalysts to drive chemical reactions transforming captured CO₂ into valuable products. Common reactions include methanation, methanol synthesis and Fischer-Tropsch synthesis, which produce sustainable aviation fuels (SAF), alternative naphtha and other hydrocarbons. Catalysts such as nickel or cobalt are employed to enhance reaction rates and selectivity.
Direct use of electricity in electrochemical CO2 reduction bypasses the need for hydrogen as an energy carrier, eliminating energy losses from water electrolysis. The process begins when CO2 molecules attach to catalytic surfaces like copper-based electrodes, where electron transfer disrupts the CO₂ structure. This breaks C=O bonds, producing adsorbed intermediates such as *CO and *COOH, which are essential for forming multi-carbon compounds. In the next step, these intermediates can combine via carbon-carbon coupling to form multi-carbon products like ethylene (C₂H₄) or ethanol (C₂H₅OH) or again form a syngas. Catalysts such as copper, palladium, or nickel enhance reaction efficiency by guiding these pathways.
Biotechnological utilisation of CO2
Biotechnological utilisation of CO₂ involves the use of microorganisms and bioengineered systems to convert carbon dioxide into valuable products, such as biomass, fuels and chemicals, by using CO2 as a carbon source instead of sugars. These processes often integrate renewable energy and biological pathways to create sustainable carbon cycles. While additional energy carriers are needed for CO2reduction, these are incorporated into microorganisms’ metabolic pathways. This enables the production of different molecules, including alcohols, organic acids and others.
Some methods combine CO₂ capture with microbial conversion in a single system to minimize energy demands, while others use renewable electricity to directly supply electrons for CO₂ reduction through microbial electrosynthesis. Photosynthetic organisms like microalgae and cyanobacteria utilise sunlight to convert CO₂ into biomass, such as lipids, carbohydrates, and proteins, via photosynthesis. These biomaterials can then be harvested for biofuels (e.g., biodiesel, ethanol) or high-value chemicals (e.g., carotenoids).
Emission reduction by CCU
The production of petrochemical raw materials is associated with high emissions. However, using CO₂ from these emissions as a feedstock can support the circular economy by closing carbon cycles and preventing additional emissions from entering the atmosphere. In this way, utilising CO2 as a raw material not only reduces the carbon footprint of many products but also replaces fossil-based materials.
Carbon-based fuels such as kerosene are essential in mobility, particularly in aviation, due to their high energy density. Despite their importance, their fossil origins result in considerable CO₂ emissions. Synthetic fuels made from captured CO₂, often referred to as e-fuels or sustainable aviation fuels (SAF), offer a viable alternative. These fuels are produced by combining captured CO₂ with green hydrogen, typically using synthesis gas as an intermediate product. Synthetic fuels are virtually free of sulphur components and can be used in existing combustion engines without major modifications, making them particularly interesting for long-distance transportation and aviation, where electrification remains limited. However, their production is energy-intensive, underscoring the importance of renewable energy sources for ensuring sustainable production.
CO₂-based Fuels and Chemicals Conference 2025

The CO₂-Based Fuels and Chemicals Conference is the leading event dedicated to CO₂ utilisation, focusing on innovative ways to transform CO₂ into valuable products. The conference highlights the use of carbon as a renewable feedstock for the chemical industry and fuel production, offering insights into cutting-edge technologies for converting CO₂ into fuels, chemicals, and materials.
Participants will explore diverse processes such as hydrogenation, Fischer-Tropsch synthesis, electrochemical methods, and biotechnological pathways to produce fuels, bulk and fine chemicals, polymers, and advanced materials like graphite for nanotubes. A dedicated session will also showcase advanced research topics, providing a glimpse into future opportunities in CO₂ utilisation.
This hybrid event will take place on 29–30 April 2025 in Cologne, Germany, and online. It offers an ideal platform for researchers, industry leaders, and policy makers to exchange ideas, learn about the latest developments in CO₂ utilisation, and network with experts from around the globe. By attending, participants can contribute to advancing sustainable solutions and help shape the future of carbon-based innovation.
Register here to join this important conversation on transforming CO₂ into a resource for a sustainable economy: https://co2-chemistry.eu/registration/
Speakers include:
- Guillermo Diaz-Sainz, University of Cantabria: Electrochemical Pathways for CO₂ Conversion: Toward Sustainable Formic Acid Production in the Cement Industry
- David Soane, Carbogenesis: Scalable and Profitable Carbon Utilisation Technology by Carbogenesis
- Dorinde Kleinegris, NORCE Norwegian Research Centre: ALGAESOL: Sustainable Aviation and Shipping Fuels from Microalgae and Direct Solar BES Technologies
- Francesca Di Bartolomeo, SINTEF: Pioneering Sustainable CO₂ Conversion to C3 Chemicals and High-Value Lipids for Feed and Food Applications
- Doris Hafenbradl, Electrochaea: Power-to-Methane: Commercially-Ready Solution for Decarbonization and Energy Storage
- Vincent Peña, Air Liquide Global E&C Solutions Germany: Saving and Valorizing CO₂ with the Lurgi MethanolTM Technologies by Air Liquide
- Oliver Kuisle, Celanese: Low Carbon Intensity Methanol from Industrial CCU
- Achim Schaadt, Fraunhofer Institute for Solar Energy Systems ISE: DME as a Promising Platform Molecule for Fuels
- Cristian Torri, Università di Bologna: Isopropyl Alcohol Production from CO₂ with a new Direct Air Carbon Capture and Fixation (DACF) Approach: Results from Early Proof-of-Concept Investigation
- Xin Tu, University of Liverpool: Plasma Catalysis for CO₂ Hydrogenation to Methanol
- Mohammad Rezaei, GIG Karasek: Exploring the Functions of Electrocatalysts and Membranes in Advanced Technologies for Green Synthesis of Carbon Monoxide from Carbon Dioxide
- Tamás Födi, eChemicles: Scaling-up Low Temperature CO₂ Electrolysis to Industrial Levels
- Philipp Arbter, Colipi: Exploiting Hydrogen-Oxidizing Bacteria as a Novel Biomanufacturing Platform for Production of Chemicals from CO₂
- Tom Wirtanen, VTT: Novel Electrosynthetic Pathways to Ethylene Glycol, Acrylic, Adipic and Glycolic acids: CO₂ based C2-C6 Building Blocks for Sustainable Polymers
For more information visit https://co2-chemistry.eu/.
Source: nova-Institute, original text, 2025-04-15.
