CO₂ recycling: What is the role of the electrolyte?
At the HZB, Matthew Mayer and his team has now investigated what causes this and how the clog up process can be prevented

The greenhouse gas carbon dioxide can be converted into useful hydrocarbons by electrolysis. The design of the electrolysis cell is crucial in this process. The so-called zero-gap cell is particularly suitable for industrial processes. But there are still problems: The cathodes clog up quickly.
The combustion of oil, coal or natural gas produces carbon dioxide, or CO2. This famous greenhouse gas is a major driver of global warming, but it is also a raw material. It is technically possible to convert CO2 into useful carbon compounds, a process which requires energy, water, suitable electrodes and special catalysts. CO2 can be electrochemically converted to carbon monoxide, formate or methane, but also to ethylene, propanol, acetate and ethanol. However, industrial processes must be designed to be highly selective and extremely efficient to produce only the desired products and not a mixture of products.
Converting CO2 back into fuel
"By electrolytically reducing CO2 to useful hydrocarbons, we can produce new fuels without using fossil resources. We thus are putting the CO2 back into the cycle, just like recycling," explains Dr Matthew Mayer, leader of the Helmholtz Young Investigator Group “Electrochemical Conversion” at HZB. The electrical energy for the electrolysis can be provided by renewable energy from wind or solar, making the process sustainable.
The zero-gap cell: a sandwich of many layers
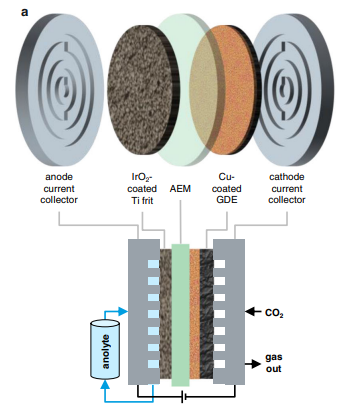
From school, we know electrolysis can be done in a simple beaker of water; a further development of this is the H-cell, which is shaped like the letter H. However, such cells are not suitable for industrial use. Instead, industrial electrolysers are designed with a sandwich architecture consisting of several layers: On the right and left are the electrodes that conduct the current and are coated with catalysts, a copper-based gas diffusion layer that lets in the CO2 gas, and a separation membrane. The electrolyte (here supplied at the anode and called anolyte) consists of dissolved potassium compounds and allows ions to move between the electrodes. The membrane is designed to allow negatively charged ions to pass through and to block positively charged potassium ions.
The problem: potassium crystals
Nevertheless, potassium ions from the electrolyte pass through the membrane and form tiny crystals at the cathode clogging the pores. "This shouldn't happen," says Flora Haun, a PhD student in Matthew Mayer's team. Using scanning electron microscopy and other imaging techniques, the scientists were able to study the process of crystal formation at the cathode in detail. "With energy-dispersive X-ray analysis, we were able to locate the individual elements and show exactly where potassium crystals were forming," Flora Haun explains.
The more potassium the electrolyte contains, the more the cathode becomes clogged, the investigations showed. But there is no simple way to solve the problem: reducing the potassium concentration is good on the one hand, but bad on the other, since the reaction equilibrium also shifts: instead of the desired ethylene, carbon monoxide is produced.
The electrolyte is the key
"The most important observation is that cations can still penetrate the anion exchange membrane, but to an extent that depends on the concentration of the electrolyte. And that with the concentration of the electrolyte we simultaneously regulate which products are formed from the CO2," says Dr. Gumaa El Nagar, a postdoctoral researcher in the team. "In the next step, we want to use operando and in situ measurements using X-rays to find out in detail how ion migration in the cell affects the chemical reaction processes," says Matthew Mayer.
Source:
Helmholtz Zentrum Berlin, press release, 2023-04-25.
