Stanford discovery could lead to sustainable source of the fuel additive ethanol
Most fuel additive ethanol used in the U.S. is made from corn, new research reveals that copper can turn carbon dioxide into ethanol without using corn or other plants
Most cars and trucks in the United States run on a blend of 90 percent gasoline and 10 percent ethanol, a renewable fuel made primarily from fermented corn. But producing the 14 billion gallons of ethanol consumed annually by American drivers requires millions of acres of farmland.
A recent discovery by Stanford University scientists could lead to a new, more sustainable way to make ethanol without corn or other crops. This technology has three basic components: water, carbon dioxide and electricity delivered through a copper catalyst. The results are published in Proceedings of the National Academy of Sciences.

Associate Professor Thomas Jaramillo (left) and SLAC scientist Christopher Hahn have demonstrated the feasibility of designing copper catalysts that convert carbon dioxide into ethanol without corn or other crops. (Image credit: Mark Shwartz/Stanford University)
“One of our long-range goals is to produce renewable ethanol in a way that doesn’t impact the global food supply,” said study principal investigator Thomas Jaramillo, an associate professor of chemical engineering at Stanford and of photon science at the SLAC National Accelerator Laboratory.
“Copper is one of the few catalysts that can produce ethanol at room temperature,” he said. “You just feed it electricity, water and carbon dioxide, and it makes ethanol. The problem is that it also makes 15 other compounds simultaneously, including lower-value products like methane and carbon monoxide. Separating those products would be an expensive process and require a lot of energy.”
Scientists would like to design copper catalysts that selectively convert carbon dioxide into higher-value chemicals and fuels, like ethanol and propanol, with few or no byproducts. But first they need a clear understanding of how these catalysts actually work. That’s where the recent findings come in.
Copper crystals
For the PNAS study, the Stanford team chose three samples of crystalline copper, known as copper (100), copper (111) and copper (751). Scientists use these numbers to describe the surface geometries of single crystals.
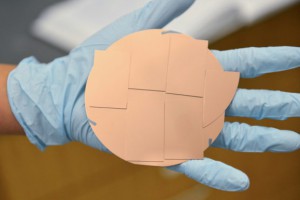
Stanford scientists have designed a large copper catalyst that produces ethanol from carbon dioxide and water. (Image credit: Mark Shwartz/Stanford University)
“Copper (100), (111) and (751) look virtually identical but have major differences in the way their atoms are arranged on the surface,” said Christopher Hahn, an associate staff scientist at SLAC and co-lead lead author of the study. “The essence of our work is to understand how these different facets of copper affect electrocatalytic performance.”
In previous studies, scientists had created single-crystal copper electrodes just 1-square millimeter in size. For this study, Hahn and his co-workers at SLAC developed a novel way to grow single crystal-like copper on top of large wafers of silicon and sapphire. This approach resulted in films of each form of copper with a 6-square centimeter surface, 600 times bigger than typical single crystals.
Catalytic performance
To compare electrocatalytic performance, the researchers placed the three large electrodes in water, exposed them to carbon dioxide gas and applied a potential to generate an electric current.
The results were clear. When the team applied a specific voltage, the electrodes made of copper (751) were far more selective to liquid products, such as ethanol and propanol, than those made of copper (100) or (111).
Ultimately, the Stanford team would like to develop a technology capable of selectively producing carbon-neutral fuels and chemicals at an industrial scale.

SLAC scientist Christopher Hahn sees his reflection in a shiny copper catalyst that converts carbon dioxide into ethanol. (Image credit: Mark Shwartz)
“The eye on the prize is to create better catalysts that have game-changing potential by taking carbon dioxide as a feedstock and converting it into much more valuable products using renewable electricity or sunlight directly,” Jaramillo said. “We plan to use this method on nickel and other metals to further understand the chemistry at the surface. We think this study is an important piece of the puzzle and will open up whole new avenues of research for the community.”
Jaramillo also serves as deputy director of the SUNCAT Center for Interface Science and Catalysis, a partnership of the Stanford School of Engineering and SLAC. Study authors include co-lead author Toru Hatsukade, Drew Higgins and Stephanie Nitopi at Stanford; Youn-Geun Kim at SLAC; and Jack Baricuatro and Manuel Soriaga at the California Institute of Technology.
Funding was provided by the U.S. Department of Energy and the Stanford Global Climate and Energy Project.
Journal Reference
Christopher Hahn, Toru Hatsukade, Youn-Geun Kim, Arturas Vailionis, Jack H. Baricuatro, Drew C. Higgins, Stephanie A. Nitopi, Manuel P. Soriaga, Thomas F. Jaramillo. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proceedings of the National Academy of Sciences, 2017; 114 (23): 5918 DOI: 10.1073/pnas.1618935114
Source: Stanford University, press release, 2017-06-16.
