Not Your Average Refinery
Review led by PNNL researchers offers a vision of sustainable energy production through electrochemical reduction
Small stockpiles of biomass such as sewage, food waste, and wood chips are often overlooked in visions of future sustainable fuel and chemical production. The dismissal arises because transporting these materials to a large-scale, centralized bio-refinery would require more energy than they produce. Yet there is enough carbon stranded in these materials to theoretically provide about 25 percent of the U.S. demand for transportation fuel.
A new review, led by researchers at Pacific Northwest National Laboratory (PNNL), offers a solution to capture these unused materials: mini-refineries located near waste sources that process biomass using electrochemical reduction reactions powered by renewable energy.
In the paper, which was recently published in Chemical Reviews, the researchers gather information from over 100 years of chemistry on theory, materials, and reactor design needed for mini-refineries to be efficient parts of industrial biomass processing.
“This work outlines a conceptual framework needed to move centuries of research into real-world applications,” said PNNL computational scientist Roger Rousseau, who leads the laboratory’s Chemical Transformations Initiative. For the past four years, the initiative has been researching fundamental electrochemistry, catalyst design, and reactor design needed for functional mini-refineries.
Electrochemical reduction produces cleaner products from bio-crude
The challenge with converting sewage, food waste, and plant waste to fuel is the necessary molecular transformations. The first step in this conversion involves decomposing the biomass under high temperature to produce a crude bio-oil. This oil contains molecules such as aldehydes, ketones, esters, acids, and phenols that contain many oxygen atoms.
Fuel, however, is made of various hydrocarbon molecules, which contain more hydrogen than oxygen.
Adding hydrogen to oxygen-rich molecules requires chemical transformations called reduction reactions. To do these reactions on bio-oil, existing industrial processes bombard the bio-crude with hydrogen gas at high temperature and pressure.
At large scales, heat produced during these reactions is captured and reused in other refining steps. This maximizes the overall energy efficiency of the process. At small scales, however, that heat is lost and not reused. This means other approaches to reduction reactions are needed for local processing of wastes on small scales.
Well-known electrochemical reduction reactions are one path toward milder conditions needed for energy-efficient mini-refineries. In these reactions, electricity and a metal catalyst, rather than hydrogen gas and heat, propel the molecular transformations. Other molecules in the mixture can also be simultaneously scavenged to provide hydrogen atoms during the reaction.
Compared to thermochemical reduction with hydrogen gas, electrochemical reductions of specific molecules in bio-oil can happen faster without increasing the reaction temperature and produce fewer byproducts. This means that fewer purification steps are needed later in production, which improves the energy efficiency of the whole process.
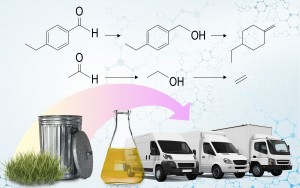
Illustration credit: Nathan Johnson | PNNL
Multidisciplinary fundamental science informing industrial applications
The fundamental electrochemistry needed for electrochemical transformations has been known for centuries. Most of that work, however, has involved laboratory studies of model compounds that represent molecules derived from biomass.
In this review, the researchers outline the information available—and still needed—to move these reactions out of the lab. That information includes research developing new catalysts that can handle complex mixtures of molecules found in bio-oil, as well as electrochemical analysis to develop energy-efficient processes.
“This review shows the capability of electrochemical conversions for bio-oil processing and shows how to optimize the reactions so they can be used beyond proof-of-principle demonstrations,” said PNNL computational scientist Vanda Glezakou.
The Chemical Transformations Initiative at PNNL provides a one-of-a-kind opportunity to advance this work because it unites researchers with expertise in catalysis with researchers specializing in electrochemistry. Together, these varied perspectives bring knowledge about the fundamental principles guiding every step of an electrocatalytic reaction. The researchers can then build upon this broad foundation to advance existing science toward applications and match specific reactions to specific production steps.
“We’ve learned that processing biomass on a local scale can contribute to sustainable fuel and chemical production,” Glezakou said. “Multidisciplinary science that we pursue at PNNL provides a holistic insight to help understand which chemical transformations are most appropriate for specific steps in an industrial scale process.”
The Chemical Transformations Initiative at PNNL is funded by the Laboratory Directed Research and Development Program.
Review co-authors include Sneha A. Akhade, PNNL and Lawrence Livermore National Laboratory; Nirala Singh, PNNL and the University of Michigan; Oliver Y. Gutiérrez, PNNL; Juan Lopez-Ruiz, PNNL; Huamin Wang, PNNL; Jamie D. Holladay, PNNL; Yue Liu, TU München; Abhijeet Karkamkar, PNNL; Robert S. Weber, PNNL; Asanga B. Padmaperuma, PNNL; Mal-Soon Lee, PNNL; Greg A. Whyatt, PNNL; Michael Elliott, PNNL; Johnathan E. Holladay, PNNL; Jonathan L. Male, PNNL; and Johannes A. Lercher, PNNL and TU München.
Source: Pacific Northwest National Laboratory (PNNL), press release, 2020-09-17.
